Degenerative Myelopathy (DM): Disease Overview and Benefits of Combined Rehabilitation Therapies
Canine degenerative myelopathy (DM) is a hereditary fatal progressive neurodegenerative disease that affects the spinal cord of adult dogs. The prognosis is poor with most dogs euthanized 6-12 months after recognition of early clinical signs. There is a breed predisposition to DM with German shepherds being the most affected, followed by Pembroke Welsh corgis, and boxers 1. DM involves mutations in the superoxide dismutase 1 gene (SOD1) that is suggested to be recessive genetic factor 2. This gene is also involved in familiar ALS disease in humans making DM a useful model for the study of human ALS 3.
The average onset age for canine degenerative myelopathy is around nine 4. Progression of disease seems to involve muscle atrophy, followed by sensory loss, and then motor neuron loss. There is no pain associated with the neurodegeneration, but pain can occur secondary to the direct effects of DM. Clinical presentation in the early disease stage is general proprioceptive ataxia with some spastic paresis in the pelvic limbs. Spinal reflex irregularities can be similar to those seen with UMN paresis from the T3-L3 spinal cord segment. Dogs can become non-ambulatory and paraparetic 5. Many pet owners will choose euthanasia at this stage making later disease stages more difficult to characterize 1. The late stages of DM include flaccid tetraplegia with LMN signs and loss of muscle mass. Dogs develop difficulty in swallowing along with respiratory dysfunction and eventual respiratory failure resulting from non-functional intercostal muscles 6.
atrophy, followed by sensory loss, and then motor neuron loss. There is no pain associated with the neurodegeneration, but pain can occur secondary to the direct effects of DM. Clinical presentation in the early disease stage is general proprioceptive ataxia with some spastic paresis in the pelvic limbs. Spinal reflex irregularities can be similar to those seen with UMN paresis from the T3-L3 spinal cord segment. Dogs can become non-ambulatory and paraparetic 5. Many pet owners will choose euthanasia at this stage making later disease stages more difficult to characterize 1. The late stages of DM include flaccid tetraplegia with LMN signs and loss of muscle mass. Dogs develop difficulty in swallowing along with respiratory dysfunction and eventual respiratory failure resulting from non-functional intercostal muscles 6.
DM is a difficult disease to diagnose and complete clinical trials on because the final diagnosis requires postmortem histopathologic examination of the spinal cord that demonstrates axonal degeneration in the white matter within the spinal cord including ascending sensory and descending motor pathways 1. This axonal degeneration is most prevalent in the caudal half of the thoracic spinal cord 7. Peripheral nerve pathology is also seen as the disease progresses and muscle pathology is consistent with denervation atrophy 3. Because owners choose to euthanize dogs at different clinical stages of the disease, and some do not choose to do the histopathology the data is not easy to standardize 1.
Current clinical studies for the treatment of canine degenerative myelopathy are focused on multimodal treatments that will slow the progression of the disease to increase both the pet's quality of life and time before euthanasia is chosen. The results of a few recent studies are summarized below.
- Physiotherapy has been shown to increase the survival time of DM dogs. In a study with 22 DM dogs those with intensive physiotherapy had a survival rate of 8 months, whereas the group of moderate physiotherapy had an average survival rate of 4 months, and those with no physical therapy had an average survival of 2 months8. Another study showed that 15-20% of the dogs having intensive physiotherapy did show a decrease in neurological state and some dogs lived for greater than four years 9.
- Photobiomodulation (PBMt) dosing for the treatment of DM was analyzed in a retrospective study. The high dose of 14-21 J/cm2 at a wavelength of 980 nm was compared to a low dose of 8 J/cm2 at a wavelength of 904 nm. With both groups undergoing the same physical rehabilitation plan along with the respective PBMt dose, the high-dose group demonstrated a significantly longer time between the onset of clinical symptoms and euthanasia an average of 38.3 months 10.
- The therapeutic value for curcumin supplementation of approximately 30 mg per day to DM patients was shown to significantly increase survival time. In this study the treatment group was small, only 6 dogs of the total 40. Curcumin has been shown to have a neuroprotective effect on the CNS as well as positive effects limiting muscle protein degradation and this data suggests it is beneficial to DM patients 11.
- A prospective controlled study evaluated DM dogs that were treated with both intensive neurorehabilitation and transplantation of mesenchymal cells. Allogenic synovial membrane stem cells were used and intrathecal transplantation was into the subarachnoid space cranial to the wings of the atlas. The sample size was a total of 13 dogs with the treatment group of 8, the inclusion criteria was non-ambulatory with a homozygous genotype. The treatment group was hospitalized for a total of 2 months, 1 month before treatment and one-month post, whereas the control group was under ambulatory treatment. The treatment group did have an increased survival time with no harmful side effects indicating a potential for stem cell transplantation to delay progression and increase the survival time of DM patients 12.
To properly determine that dogs presented with a differential diagnosis of T3-L3 spinal cord lesion have DM antemortem the veterinary practitioner must consider the dog's clinical history, and the dog must have both the absence of spinal cord compression and the presence of the SOD1 gene mutation 13. When DM patients are presented for rehabilitation, a proper multi-modal program helps these dogs maintain mobility longer, have a better quality of life for a longer period of time, and can extend their life expectancy 8-12.
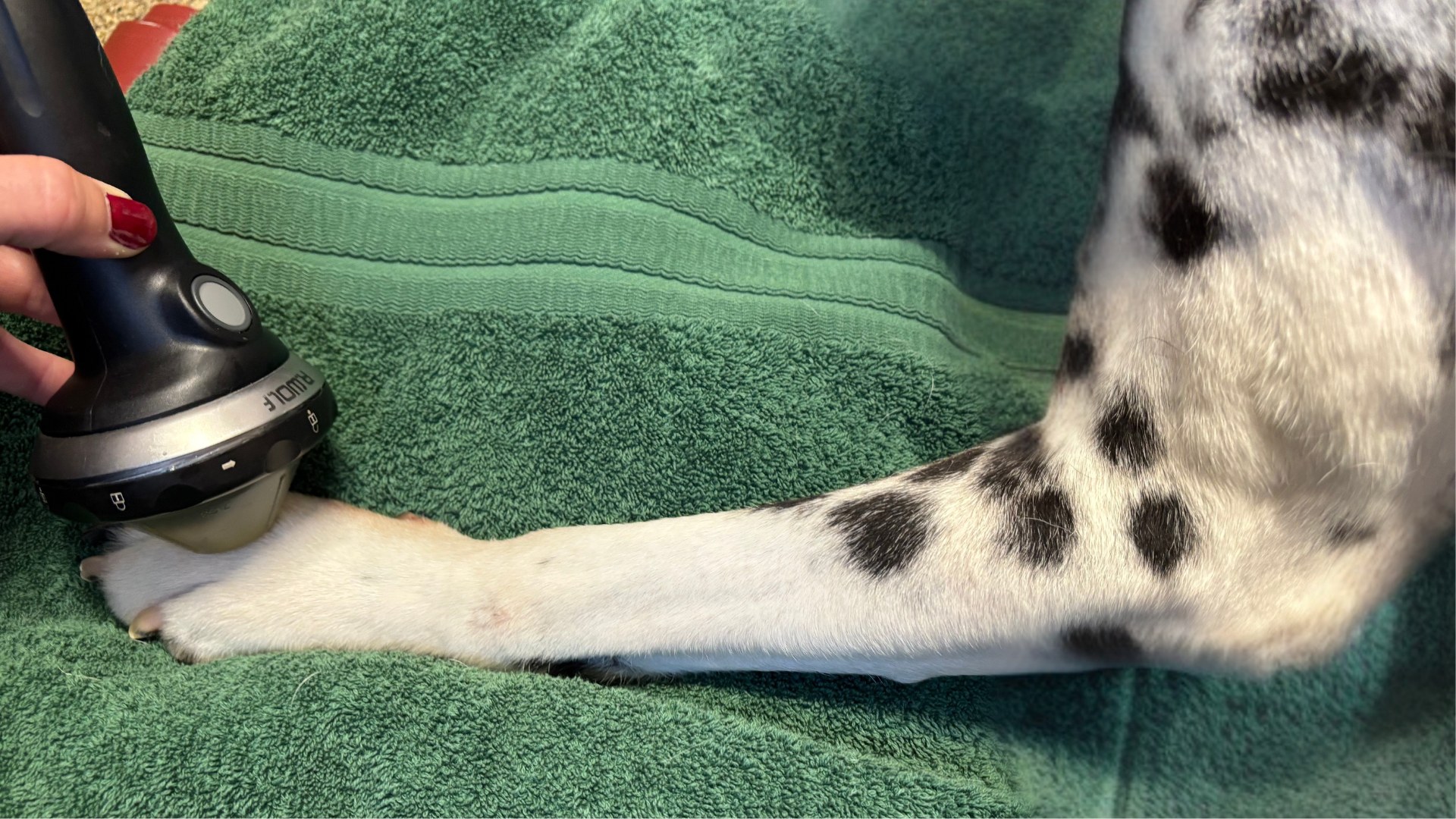
There is anecdotal evidence that the addition of low-energy PiezoWave2T Vet shockwave therapy to a multimodal rehabilitation plan for DM patients can help maintain mobility, improve quality of life, and potentially increase survival time. Shockwave therapy is effective for managing pain including neuropathic pain and pain from spasticity or cramps 14,15. It also treats conditions secondary to DM such as prolonged immobility causing degenerative changes in joints and connective tissue occurring with the progression of muscle weakness and atrophy 16. Therapeutic shockwave mechanisms that may support these positive effects include peripheral nerve regeneration, angiogenesis, VEGF expression, and a decrease in muscle atrophy 17-19. When treating DM patients with PiezoWave2T Vet shockwave, the energy is applied to the paraspinal muscles and peripheral nerves after they exit the spinal cord. ELvationUSA Vet advises against treating the spinal cord directly. Although more research is needed to validate shockwave therapy for canine degenerative myelopathy, it is a safe therapy for peripheral nerves and paraspinal muscles. Learn more about shockwave therapy around the spine in this blog post. PiezoWave2T Vet shockwave offers multiple potential benefits as a non-invasive therapy for DM patients undergoing rehabilitation.
References
- Coates, J. R., March, P. A., Oglesbee, M., Ruaux, C. G., Olby, N. J., Berghaus, R. D., ... & Williams, D. A. (2007). Clinical characterization of a familial degenerative myelopathy in Pembroke Welsh Corgi dogs. Journal of Veterinary Internal Medicine, 21(6), 1323-1331.
- Zeng, R., Coates, J. R., Johnson, G. C., Hansen, L., Awano, T., Kolicheski, A., ... & Johnson, G. S. (2014). Breed distribution of SOD 1 alleles previously associated with canine degenerative myelopathy. Journal of veterinary internal medicine, 28(2), 515-521.
- Nardone, R., Höller, Y., Taylor, A. C., Lochner, P., Tezzon, F., Golaszewski, S., ... & Trinka, E. (2016). Canine degenerative myelopathy: a model of human amyotrophic lateral sclerosis. Zoology, 119(1), 64-73.
- Coates, J. R., & Wininger, F. A. (2010). Canine degenerative myelopathy. Veterinary Clinics: Small Animal Practice, 40(5), 929-950.
- Morgan, B. R., Coates, J. R., Johnson, G. C., Shelton, G. D., & Katz, M. L. (2014). Characterization of thoracic motor and sensory neurons and spinal nerve roots in canine degenerative myelopathy, a potential disease model of amyotrophic lateral sclerosis. Journal of neuroscience research, 92(4), 531-541.
- Oyake, K., Kobatake, Y., Shibata, S., Sakai, H., Saito, M., Yamato, O., ... & Kamishina, H. (2016). Changes in respiratory function in Pembroke Welsh Corgi dogs with degenerative myelopathy. Journal of Veterinary Medical Science, 78(8), 1323-1327.
- March, P. A., Coates, J. R., Abyad, R. J., Williams, D. A., O'brien, D. P., Olby, N. J., ... & Oglesbee, M. (2009). Degenerative myelopathy in 18 Pembroke Welsh corgi dogs. Veterinary pathology, 46(2), 241-250.
- Kathmann, I., Cizinauskas, S., Doherr, M. G., Steffen, F., & Jaggy, A. (2006). Daily controlled physiotherapy increases survival time in dogs with suspected degenerative myelopathy. Journal of veterinary internal medicine, 20(4), 927-932.
- Polizopoulou, Z., Koutinas, A., Patsikas, M., & Soubasis, N. (2008). Evaluation of a proposed therapeutic protocol in 12 dogs with tentative degenerative myelopathy. Acta Veterinaria Hungarica, 56(3), 293-301.
- Miller, L. A., Torraca, D., & De Taboada, L. (2020). Retrospective observational study and analysis of two different photobiomodulation therapy protocols combined with rehabilitation therapy as therapeutic interventions for canine degenerative myelopathy. Photobiomodulation, photomedicine, and laser surgery, 38(4), 195-205.
- Kobatake, Y., Nakata, K., Sakai, H., Sasaki, J., Yamato, O., Takashima, S., ... & Kamishina, H. (2021). The long-term clinical course of canine degenerative myelopathy and therapeutic potential of curcumin. Veterinary Sciences, 8(9), 192.
- Gouveia, D., Correia, J., Cardoso, A., Carvalho, C., Oliveira, A. C., Almeida, A., ... & Martins, Â. (2023). Intensive neurorehabilitation and allogeneic stem cells transplantation in canine degenerative myelopathy. Frontiers in Veterinary Science, 10, 1192744
- Bouché, T. V., Coates, J. R., Moore, S. A., Faissler, D., Rishniw, M., & Olby, N. J. (2023). Diagnosis and management of dogs with degenerative myelopathy: A survey of neurologists and rehabilitation professionals. Journal of Veterinary Internal Medicine, 37(5), 1815-1820.
- Nedelka, T., Nedelka, J., Schlenker, J., Hankins, C., & Mazanec, R. (2014). Mechano-transduction effect of shockwaves in the treatment of lumbar facet joint pain: comparative effectiveness evaluation of shockwave therapy, steroid injections and radiofrequency medial branch neurotomy. Neuroendocrinol Lett, 35(5), 393-7.
- Martínez, I. M., Sempere-Rubio, N., Navarro, O., & Faubel, R. (2020). Effectiveness of shock wave therapy as a treatment for spasticity: a systematic review. Brain Sciences, 11(1), 15.
- Simplicio, C. L., Purita, J., Murrell, W., Santos, G. S., Dos Santos, R. G., & Lana, J. F. S. D. (2020). Extracorporeal shock wave therapy mechanisms in musculoskeletal regenerative medicine. Journal of Clinical Orthopaedics and Trauma, 11, S309-S318.
- Sagir, D., Bereket, C., Onger, M. E., Bakhit, N., Keskin, M., & Ozkan, E. (2019). Efficacy of extracorporeal shockwaves therapy on peripheral nerve regeneration. Journal of Craniofacial Surgery, 30(8), 2635-2639.
- Yahata, K., Kanno, H., Ozawa, H., Yamaya, S., Tateda, S., Ito, K., ... & Itoi, E. (2016). Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. Journal of Neurosurgery: Spine, 25(6), 745-755.
- Lee, J. H., & Cho, S. H. (2013). Effect of extracorporeal shock wave therapy on denervation atrophy and function caused by sciatic nerve injury. Journal of physical therapy science, 25(9), 1067-1069.
ELvation Marketing Team
Combining sales and flexible customer support with many years’ in-depth knowledge of medical equipment we offer customized solutions to create value with long-term investments and medical supplies. ELvation’s strength lies in its ability to combine the apparently contradictory needs of improving the standards of patient care by providing high-quality medical technology and good corporate profitability. We have a special partnership with Richard Wolf GmbH as their long-term authorized Sales & Service Team for piezo shockwave systems.